Happy Malaysia Day (16th September 2019) to all Malaysians! On behalf of InterVenn Biosciences (InterVenn), I wish all Malaysians unity, prosperity, and importantly, happiness and good health! On this auspicious day, I would like to take some time to reflect on InterVenn’s efforts and contributions to women’s healthcare in Malaysia, and to reaffirm our commitment to reducing the burden of ovarian cancer, and improving the health and wellbeing of all Malaysian women.
Ovarian cancer is the fourth most common cancer among women in Malaysia. Unfortunately, due to the lack of effective tools for accurate early detection and confirmatory diagnosis, most women are diagnosed at an advanced stage, when the disease is less curable. In an effort to address this escalating problem, InterVenn (HQ in Redwood City, California) has established a strategic partnership with Clinical Research Malaysia (CRM) to conduct the VOCAL (Venn Ovarian Cancer Liquid Biopsy) clinical study in Malaysia, strengthening the capacity of our clinical studies as well as applicability of our study results in Southeast Asia, especially Malaysia.
The VOCAL study is one of the largest global ovarian cancer clinical trials, spanning over three countries and two continents. Through our collaboration with CRM, InterVenn successfully launched the VOCAL study in Malaysia in May 2019. As of 31st August 2019, which celebrates Malaysia’s Independence Day, the VOCAL study has enrolled a total of 110 patients in Southeast Asia, 63 of whom were enrolled through the 8 active sites in Malaysia.
InterVenn is grateful for the opportunity to partner with CRM, and would like to thank all of our study PIs and study teams across Malaysia for the continued support and persistent hard work. In particular, we would like to thank the study team at Hospital Wanita dan Kanak-kanak Sabah, which was activated on 11th July 2019 and has thus far enrolled 8 participants with complete EDC data. We would also like to extend a special thank you to the study team at Hospital Seberang Jaya, the first initiated site in Malaysia, for being proactive and helping us overcome many initial challenges. We look forward to a successful VOCAL study in Malaysia, and to share exciting study announcements and updates in the future!
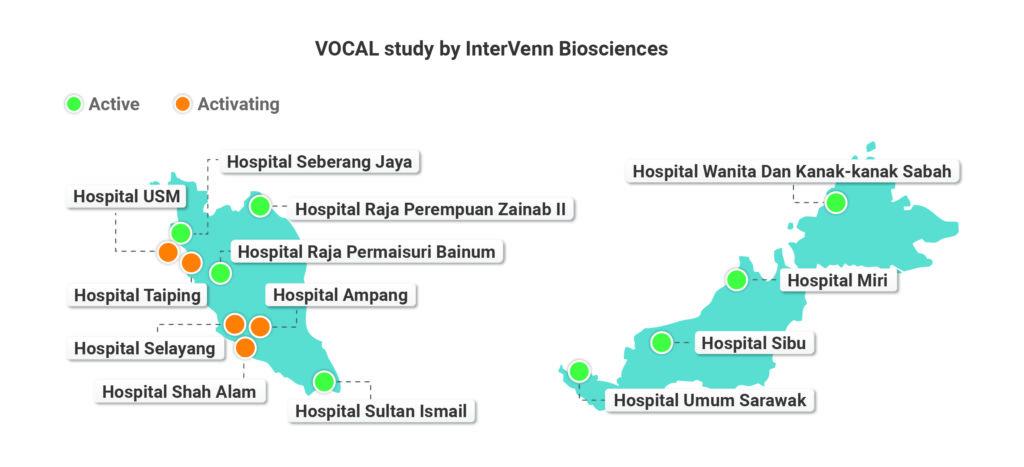
Top Three Enrolling Sites in Malaysia As of 31 August, 2019:
| Hospital Sultan Ismail Dr Nor Hidayah bt Yayanto (PI), Dr Maizatulernawani Hashim (Sub-I), Rathi a/p Raja (lead SC), Adnin Adawiyah bt Makhul (SC) | 21 |
| Hospital Seberang Jaya Dr Salina bt Sany (PI), Dr Ng Li Yun (Sub-I), Dr Stephenie Ann a/p Albart (Sub-I), Dr Norul Akhma bt Abdul Hamid (Sub-I), Francis Ooi Bee Ai (lead SC), Awatif bt Mohd Rusli (SC) | 16 |
| Hospital Raja Permaisuri Bainun Dr Lee Saw Joo (PI) , Dr Lim Xin Jie (Sub-I), Cheng Shu Xian (Sub-I), Sivanesan s/o S. Seevagan (lead SC), Wan Ainor Syahdah (SC) | 11 |
Top Three Sites with Most EDC Data Completion:
| Hospital Seberang Jaya Dr Salina bt Sany (PI), Dr Ng Li Yun (Sub-I), Dr Stephenie Ann a/p Albart (Sub-I), Dr Norul Akhma bt Abdul Hamid (Sub-I), Francis Ooi Bee Ai (lead SC), Awatif Binti Mohd Rusli (SC) |
| Hospital Umum Sarawak Dr Sim Wee Wee (PI), Dr Teh Beng Hock (Sub-I), Dr Kanddy Loo Chin Yee (Sub-I), Dr Chin Yeung Sing (Sub-I), Tan Boon Sin (lead SC), Masselleny Gay Anak Joshua Giang (SC), Ngu Lin Hui (SC), Arolyn Anak Sullong @ Alfred (SN), Loong Anak Ikok (SN) |
| Hospital Miri Dr Juliana bt Mohamad Basuni (PI), Dr Soo Kok Leong (Sub-I), Dr Nik Azi Azuha Bt Nik Hassan (Sub-I), Dr Freddie Anak Atuk (Sub-I), Dr Nor Iliana Ahmad Nordin (Sub-I), Slyvia Anak Stephen Bejit (lead SC), Salina Lisang (SC |
We thank CRM, study PIs and teams particularly the study team (PI Dr Soon Ruey, Sub-I Dr Arivendran A/L Dhorai Raja, Sub-I Dr Sofia Annaim bt Abdussyukur, Sub-I Dr Jerry Moinon, Sub-I Dr Ong Teck Siong, lead SC Hirda Wati, SC Roslin Guadih, SN Cornelia Sumporoh, SN Eve Safera Francis) at Hospital Wanita dan Kanak-kanak Sabah, which was activated on 11 Jul 2019 and by 31 Aug 2019 had a total 8 participants enrolled with prompt EDC data completion. Our appreciation also extends to the study team at Hospital Seberang Jaya, which was the first initiated site in Malaysia. Throughout the initiation process, the study team has been proactive and has helped us overcome many challenges. We look forward to having a successful VOCAL study in Malaysia and upcoming study announcements and updates. Our sincerest appreciation to all the participating hospitals for the continued support and persistent hard work. Thank you!








